
Material-enhanced Fenton-like reactions, such as heterogeneous sulfate radical (SO4•–)-based advanced oxidation processes (AOPs), are efficient technologies for water decontamination. Heterogeneous catalysts such cobalt-based catalysts are found to show high peroxymonosulfate (PMS, HSO5–) or persulfate (PS, S2O8–) activation efficiency due to the specific 3d band structure of Co2+. However, there is still a big challenge on promoting the electron transfer from a transition metal to PMS/PS.
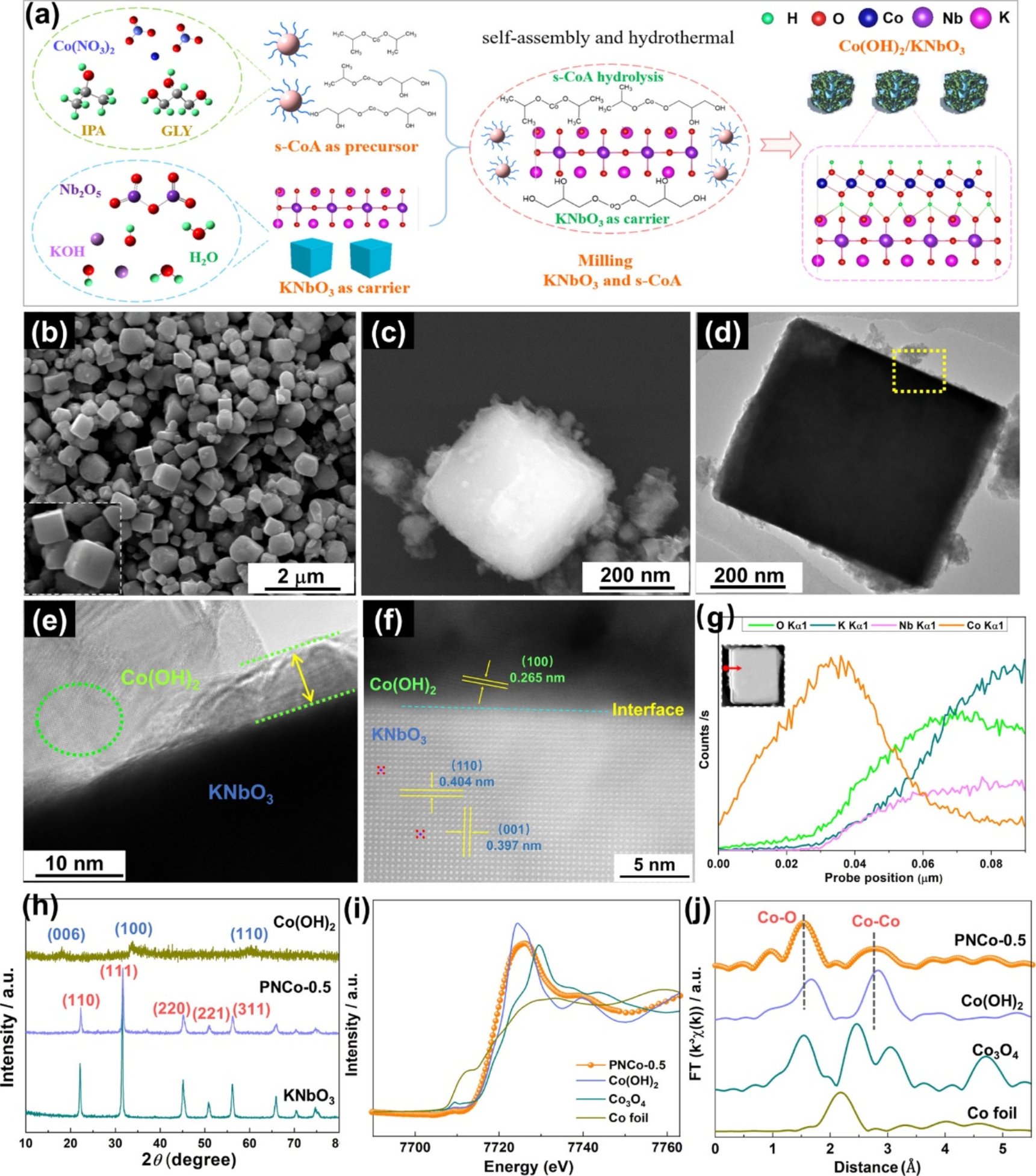
Figure 1. (a) Illustration on the synthesis process of the Co(OH)2/KNbO3 interface structure catalyst. Scanning electron microscopy (SEM) images of (b) cubic KNbO3 and (c) PNCo-0.5; (d and e) Transmission electron microscopy (TEM) and (f) high-angle annular dark-field scanning TEM (HAADF-STEM) images of PNCo-0.5; (g) Energy-dispersive spectroscopy (EDS) elemental line mapping in PNCo-0.5; (h) X-ray diffraction (XRD) patterns of KNbO3, PNCo-0.5, and Co(OH)2; (i) Co X-ray absorption near-edge structure (XANES) spectra; and (j) Co K-edge extended X-ray absorption fine structure spectroscopy (EXAFS) spectra.
Recently, Wen Liu’s group developed an interface architecture of Co(OH)2 nanosheets coated on the KNbO3 perovskite [Co(OH)2/KNbO3], which showed high catalytic activity in PMS activation. A high reaction rate constant (k1) of 0.631 min–1 and complete removal of pazufloxacin within 5 min were achieved. X-ray photoelectron spectroscopy, X-ray absorption near edge structure spectra, and density functional theory (DFT) calculations revealed the successful construction of the material interface and modulated electronic structure for Co(OH)2/KNbO3, resulting in the hole accumulation on Co(OH)2 and electron accumulation on KNbO3. Bader topological analysis on charge density distribution further indicates that the occupations of Co-3d and O-2p orbitals in Co(OH)2/KNbO3 are pushed above the Fermi level to form antibonding states (σ*), leading to high chemisorption affinity to PMS. In addition, more reactive Co(II) with the closer d-band center to the Fermi level results in higher electron transfer efficiency and lower decomposition energy of PMS to SO4•–. Moreover, the reactive sites of pazufloxacin for SO4•– attack were precisely identified based on DFT calculation on the Fukui index. The pazufloxacin pathways proceeded as decarboxylation, nitroheterocyclic ring opening reaction, defluorination, and hydroxylation. This work can provide a potential route in developing advanced catalysts based on manipulation of the interface and electronic structure for enhanced Fenton-like reaction such as PMS activation.
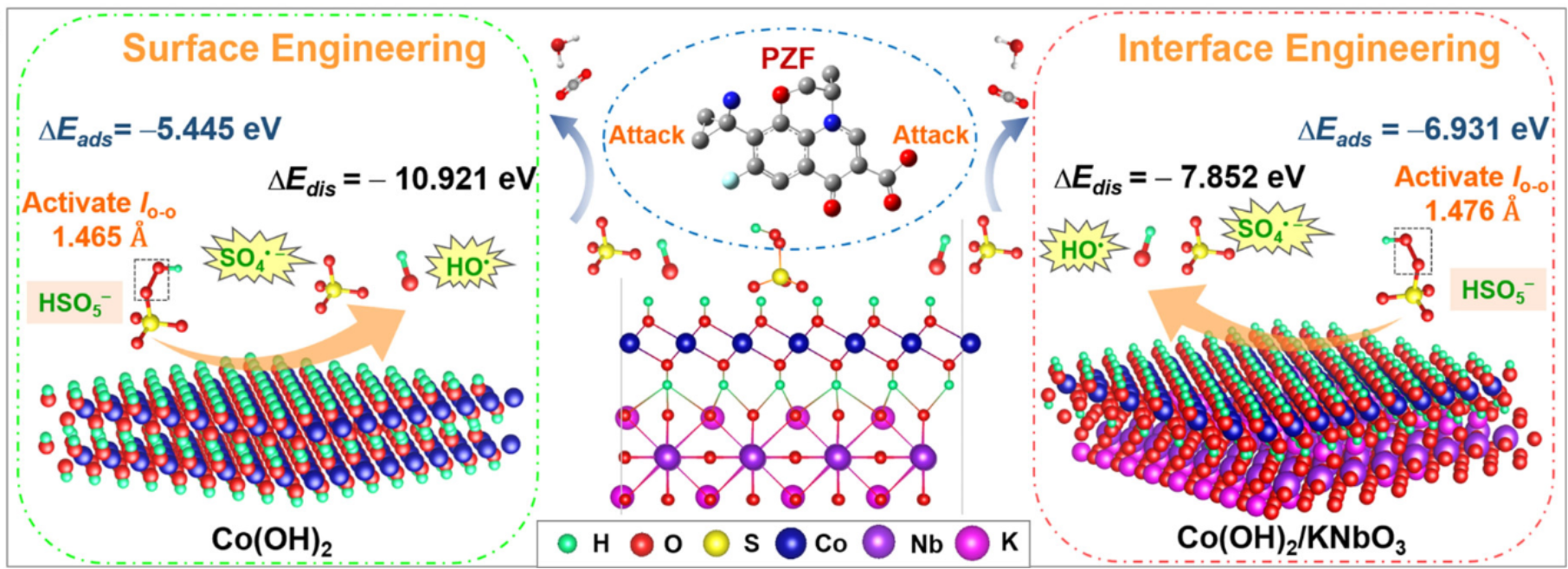
Figure 2. Illustration of the PMS activation mechanism for the surface and interface engineering and the CDD plot at the Co(OH)2/KNbO3 interface and HSO5–; the green, red, yellow, blue, violet, and purple atoms represent H, O, S, Co, Nb, and K atoms, respectively.
In the future, a possible research strategy can be considered to achieve better understanding of the mechanisms in SO4•–-based heterogeneous AOPs, including the following three steps: (1) first, a radical control method should be proposed to construct different radical systems, such as systems with different ratios of SO4•– to HO• via solution pH adjusting; (2) second, transformation products formed via attack of a specific radical should be carefully identified through high-resolution mass spectroscopy; (3) finally, theoretical calculations on free energy change, molecular orbital distribution, NPA change distribution, band cleavage and formation, and even electronic structure change in radical and organic compounds should be comprehensively conducted to reveal the underlying reaction mechanism. The findings in this work provide an available method to develop such materials in the PMS/PS-AOP system, and it is reasonable to believe that the interface engineering and DFT calculations for the catalytic enhancement mechanism would provide deep insights into the interaction between materials and emerging contaminants at the interface.
The study has been recently published online in Environmental Science and Technology as a title of “Interface Engineering of Co(OH)2 Nanosheets Growing on the KNbO3 Perovskite Based on Electronic Structure Modulation for Enhanced Peroxymonosulfate Activation” (https://doi.org/10.1021/acs.est.1c08806). The first author of the paper is Dr. Juanjuan Qi, a postdoctoral scholar from Dr. Wen Liu’s group in the College of Environmental Sciences and Engineering, Peking University. The co-first author is Dr. Xiaoyong Yang from the State Key Laboratory of Environment-friendly Energy Materials, Southwest University of Science and Technology. Dr. Wen Liu is the corresponding author. This project is supported by the National Key Research and Development Program of China (no. 2021YFA1202500), the National Natural Science Foundation of China (no. 21906001, no. 22106045, no. 11705152), and Beijing Nova Program (Z191100001119054).
Related reference
Qi, J., Yang, X., Pan, P. Y., Huang, T., Yang, X., Wang, C. C., Liu, W*. Interface engineering of Co(OH)2 nanosheets growing on the KNbO3 perovskite based on electronic structure modulation for enhanced peroxymonosulfate activation. Environmental Science & Technology, 2022, 56(8), 5200-5212.