
The hydroxyl radicals (OH radicals) are the most important oxidants in the atmosphere, determining the self-cleaning capability in the troposphere. However, the hypothesis of "unclassical OH regeneration mechanism" in urban areas and forests, proposed by Peking University and the Research Centre Juelich in Germany (Science 2009) and the Max Planck Institute for Chemistry in Germany (Nature 2008), respectively, has been an unresolved challenge in the field of international atmospheric chemistry.
Identifying the "unclassical OH regeneration mechanism" is of great significance for the precise prevention and control of secondary pollution and for addressing climate change. Although prior studies have shown that the autoxidation mechanism of isoprene contributes to OH radicals under ultra-low NOx conditions (e.g. forests), the contribution of isoprene autoxidation mechanism in urban and other high NOx areas is not significant, which could not explain the theory of "unclassical OH regeneration mechanism".
The research team led by Lu Keding constructed a dataset from seven summer and autumn radical-centered field campaigns conducted in typical urban agglomerations in China since 2006. They discovered a strong positive linear relationship between the reactivity of oxygenated volatile organic compounds (OVOCs) and the missing OH sources, inferring that OVOCs might be the key species involved in the " unclassical OH regeneration mechanism". To further verify this conjecture, this team explored the missing OH sources based on multidimensional research methods, including quantum chemical calculations and numerical simulation closure experiments. They revealed for the first time that the higher aldehyde autoxidation mechanism (HAM) is the crucial reaction pathway for the "unclassical OH regeneration mechanism". The detailed reaction pathways are as follows: higher aldehydes could be oxidized to carbonyl organic peroxy radicals (R(CO)O2), which would undergo an H-migration process to generate hydroperoxyl-carbonyls (HPC). Subsequently, HPC could rapidly regenerate OH radicals through photolysis (Figure 1).
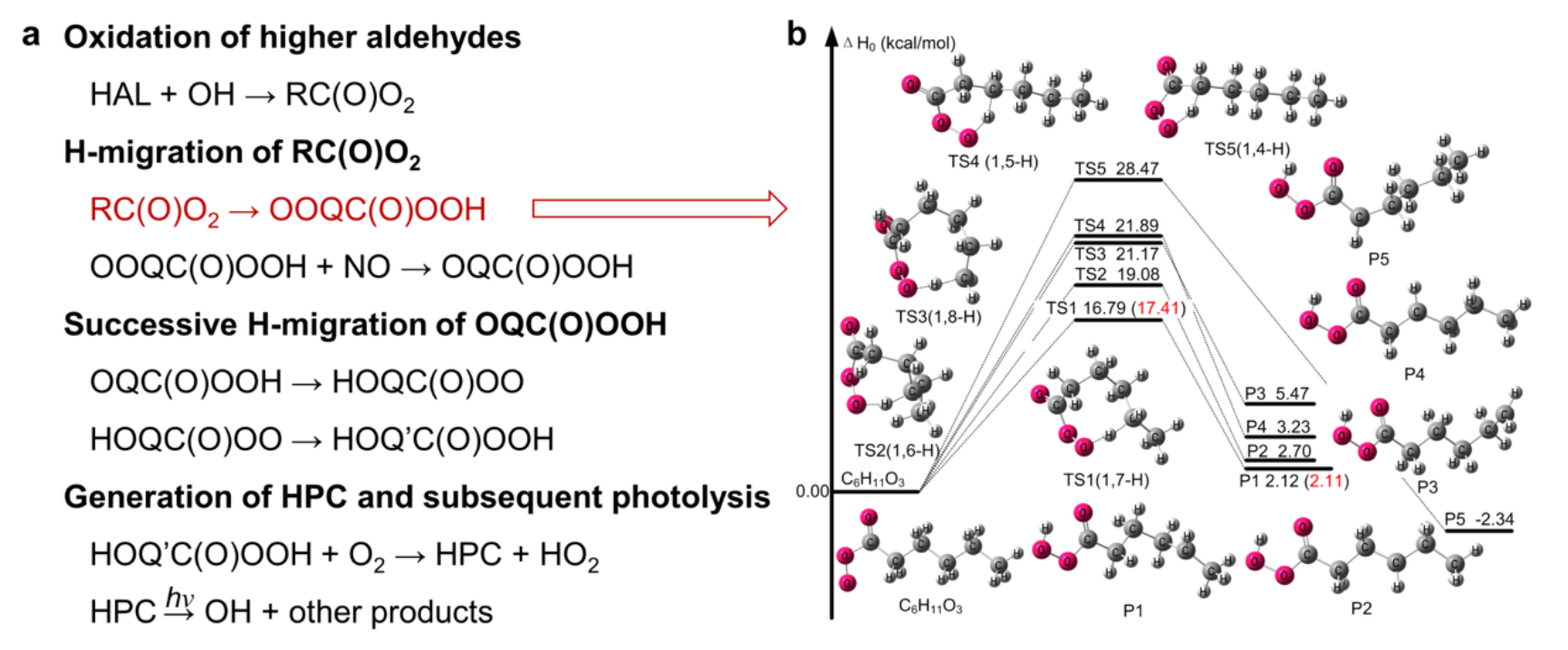
Figure 1. (a) Higher aldehyde autoxidation mechanism (HAM). (b) Energy level diagram of the H-migration reaction of RC(O)O2 radicals.
Building on this foundation, the article further proposed a generalized reactive aldehyde autoxidation mechanism (RAM), in which all R(CO)O2 radicals are extrapolated to regenerate OH radicals with different yields according to their molecular structures. Based on a comprehensive analysis from fourteen global radical-centered field campaigns, they found that RAM is a key theoretical explanation of "unclassical OH regeneration mechanism". Surprisingly, the contribution of RAM exceeds that of isoprene autoxidation mechanism in both urban areas and some forests (Figure 2).
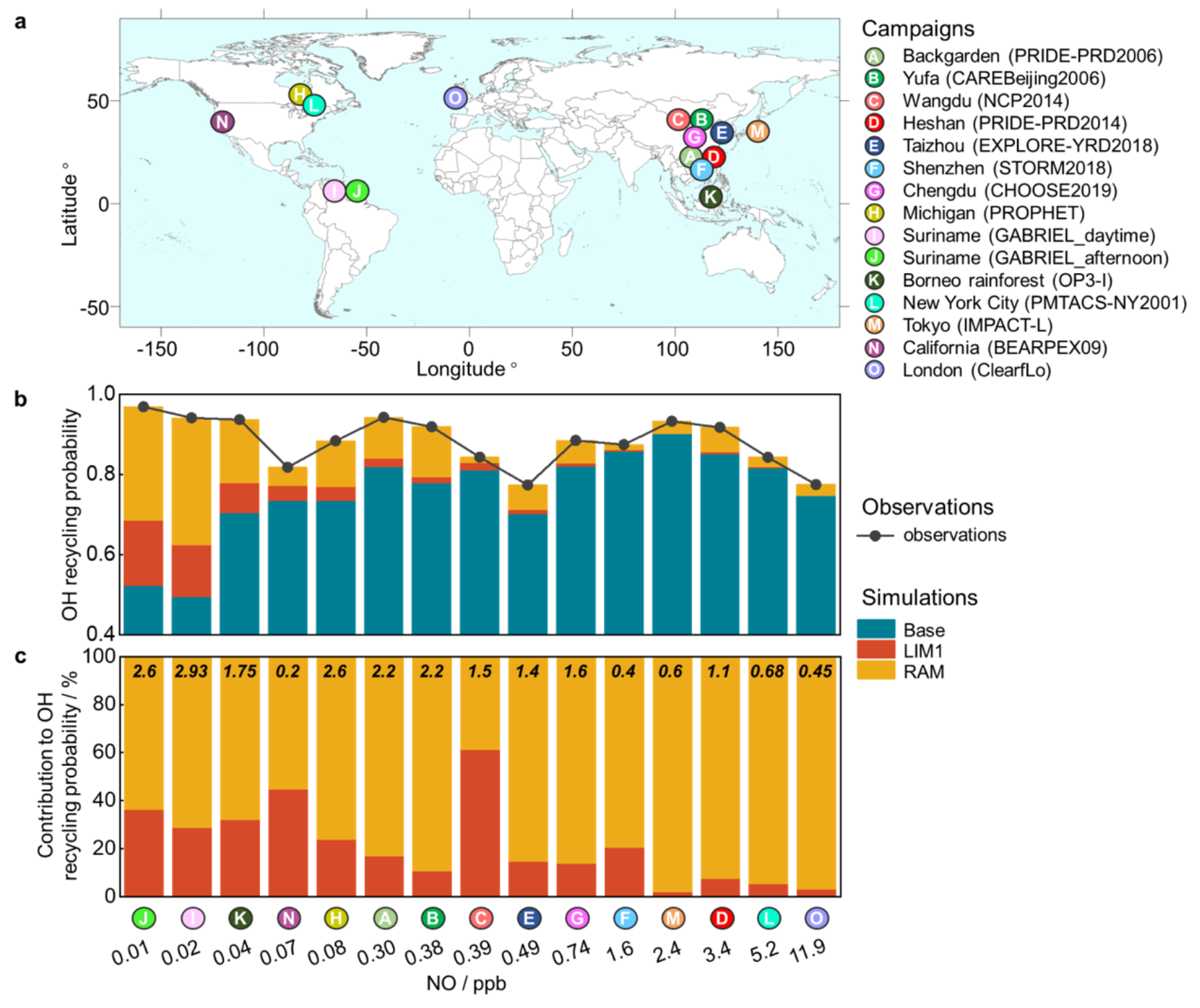
Figure 2. The impact of the isoprene autoxidation mechanism (LIM1) and reactive aldehyde autoxidation mechanism (RAM) on radical regeneration within different NO concentrations.
This article proposed a conceptual model for the OH radical regeneration mechanism (Figure 3). This model indicates that the contribution of the isoprene autoxidation mechanism to OH regeneration is concentrated in ultra-low NOx conditions (mainly forests), while the contribution of the aldehyde autoxidation mechanism to OH regeneration occurs in low NOx conditions (such as the transition zones between forests and urban areas). The classical secondary pathway (reactions of NO and HO2) dominates the OH production in high and ultra-high NOx conditions. With the rapid advancement of global carbon neutrality strategies, atmospheric NOx concentrations will significantly decrease, thus further highlighting the contribution of aldehyde autoxidation mechanism to OH regeneration and thus atmospheric oxidation capacity.
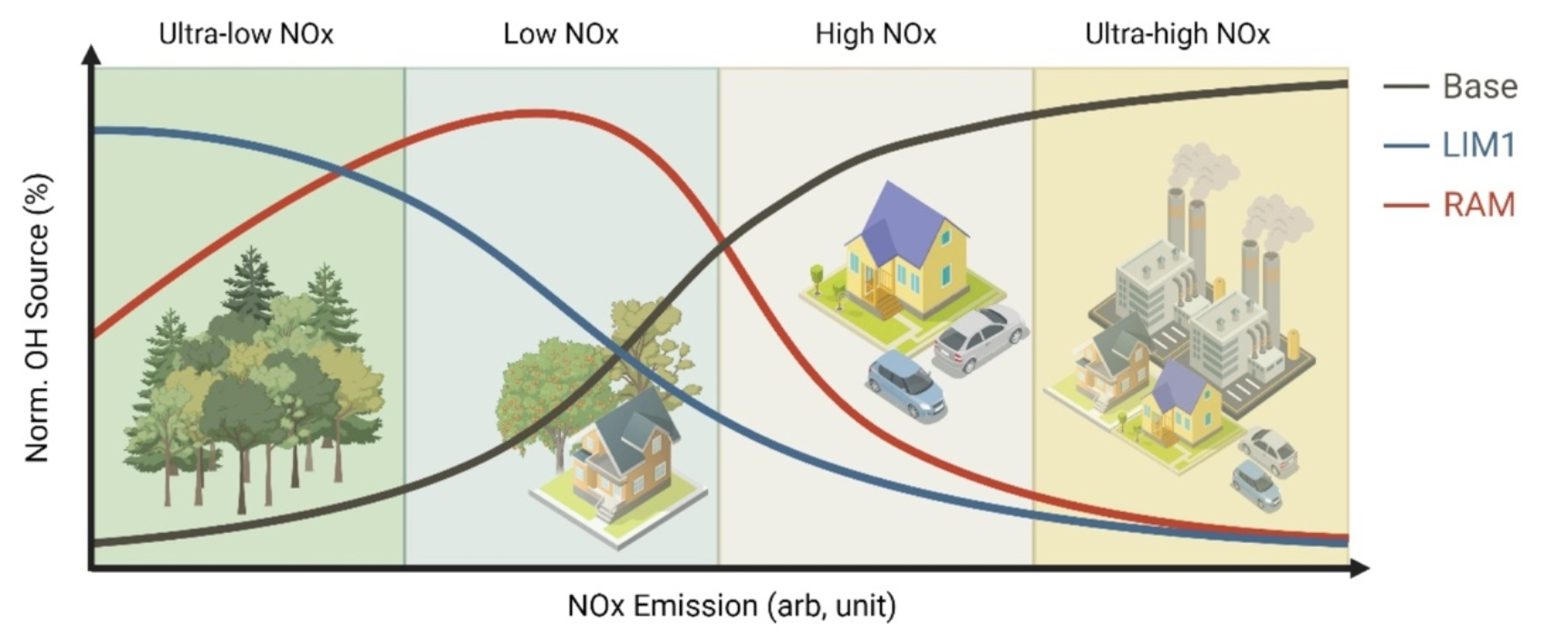
Figure 3. Conceptual model of OH radical regeneration sources under different NOx concentrations.
(Base represents the classical atmospheric chemistry mechanism, mainly regenerated through reactions with NO and HO2 radicals; LIM1 is the latest version of isoprene autoxidation regeneration mechanism; RAM is the reactive aldehyde autoxidation mechanism).
This work provides a pivotal theoretical explanation for the conceptual model underlying the non-traditional OH regeneration mechanism. It represents another remarkable and original achievement by Lu’s team in the study of atmospheric OH radical chemistry, holding critical significance for enhancing our comprehension of tropospheric chemical reaction mechanisms.
This research findings have published online in Nature Communications on February 22, 2024, titled "Reactive aldehyde chemistry explains the missing source of hydroxyl radicals." Professor Lu Keding and Academician Zhang Yuanhang from the College of Environmental Sciences and Engineering at Peking University are the corresponding authors. Assistant Researcher Yang Xinping from the Chinese Research Academy of Environmental Sciences (a 2021 Ph.D. graduate from Peking University) and Associate Professor Wang Haichao from Sun Yat-sen University (a 2018 Ph.D. graduate and Boya Postdoctoral Fellowship from Peking University) are the co-first authors. Professor Long Bo's team from Guizhou Minzu University provided invaluable support in quantum chemical calculations for this study. This study was funded by the National Science Fund for Distinguished Young Scholars and the National Key Research and Development Program of the Ministry of Science and Technology, among other projects.