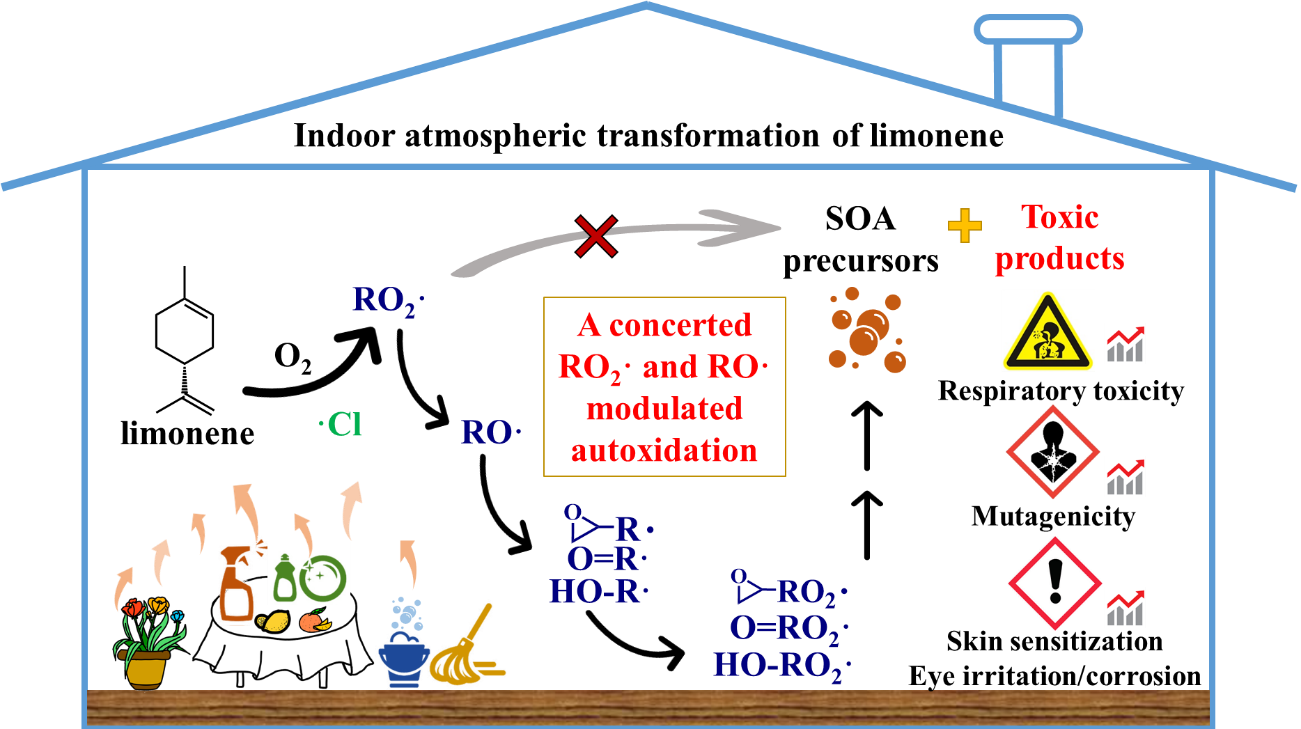
Limonene, a key volatile chemical product (VCP) commonly found in personal care and cleaning agents, is emerging as a major indoor air pollutant. Recently, elevated levels of reactive chlorine species during bleach cleaning and disinfection have been reported to increase indoor oxidative capacity. However, incomplete knowledge of the indoor transformation of limonene, especially the missing chlorine chemistry, poses a barrier to evaluating the environmental implications associated with the concurrent use of cleaning agents and disinfectants. Here, we investigated the reaction mechanisms of chlorinated limonene peroxy radicals (Cl-lim-RO2•), key intermediates in determining the chlorine chemistry of limonene, and toxicity of transformation products (TPs) using quantum chemical calculations and toxicology modeling. The results indicate that Cl-lim-RO2• undergoes a concerted autoxidation process modulated by RO2• and alkoxy radicals (RO•), particularly emphasizing the importance of RO• isomerization. Following this generalized autoxidation mechanism, Cl-lim-RO2• can produce low-volatility precursors of secondary organic aerosols. Toxicological findings further indicate that the majority of TPs exhibit increased respiratory toxicity, mutagenicity, and eye/skin irritation compared to limonene, presenting an occupational hazard for indoor occupants. The proposed near-explicit reaction mechanism of chlorine-initiated limonene significantly enhances our current understanding of both RO2• and RO• chemistry while also highlighting the health risks associated with the concurrent use of cleaning agents and disinfectants.
https://doi.org/10.1021/acs.est.4c04388