

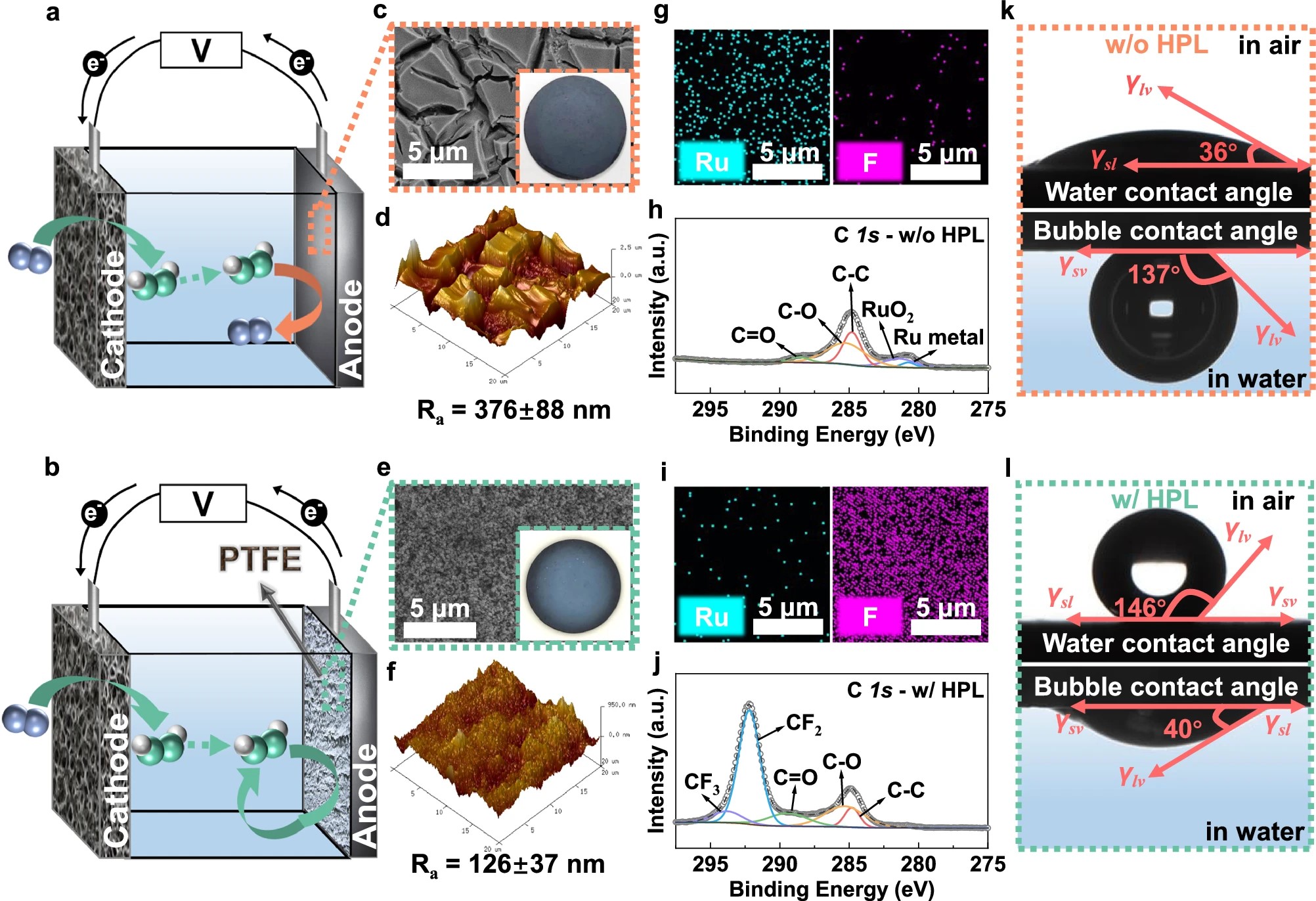
Hydrogen peroxide (H2O2) can be sustainably synthesized through the electrochemical oxygen reduction reaction in a dual-chamber water electrolyzer separated by expensive ion exchange (IX) membranes. The development of an IX membrane-free electrolyzer has been limited by direct anodic degradation of the produced H2O2. Here, we devise a bubble shielding strategy by using a low-cost polytetrafluoroethylene hydrophobic porous layer (HPL) on the anode that enables numerous sites for anodically generated oxygen bubbles and significantly suppresses H2O2 degradation in the electrolyte. The H2O2 production increases by ~600% compared to that using non-bubble shielded anode. A high H2O2 concentration of 10.05 ± 0.05 g L−1 at 40 mA cm−2 can be obtained with both HPL-coated anode and cathode. A solar-driven disinfection device equipped with HPL-coated electrodes achieves >99.9% E. coli inactivation within 60 min. This innovative approach for achieving high electrochemical H2O2 concentrations in IX membrane-free electrolyzers more generally provides insights for fine tuning three-phase interfaces and could be applicable to other reactions pathways in electrochemical applications.
https://www.nature.com/articles/s41467-025-57116-x