Xiaoning Xuan, Zhongming Chen, Yiwei Gong, Hengqing Shen, and Shiyi Chen
https://doi.org/10.5194/acp-20-5513-2020
Abstract
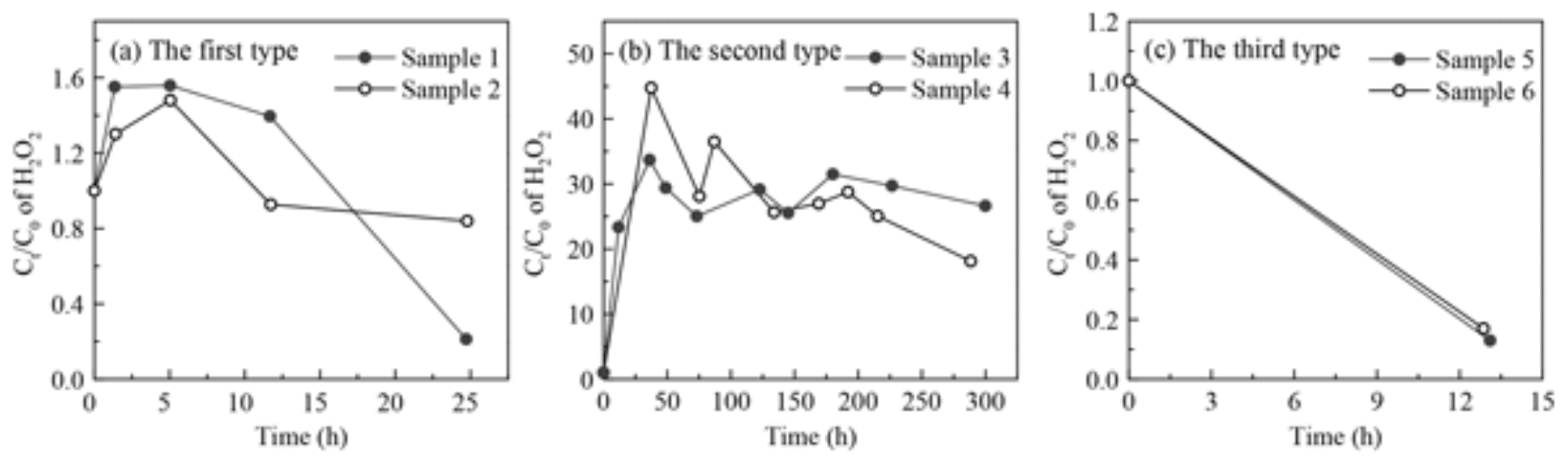
Hydrogen peroxide (H2O2) is a vital oxidant in the atmosphere and plays critical roles in the oxidation chemistry of both liquid and aerosol phases. The partitioning of H2O2 between the gas and liquid phases, or the aerosol phase, could affect its abundance in these condensed phases and eventually the formation of secondary components. However, the partitioning processes of H2O2 in gas-liquid and gas-aerosol phases are still unclear, especially in the ambient atmosphere. In this study, field observations of gas-, liquid-, and aerosol-phase H2O2 were carried out in the urban atmosphere of Beijing during the summer and winter of 2018. The effective field-derived mean value of Henry's law constant (HmA, 2.1×105 M atm−1) was 2.5 times of the theoretical value in pure water (HtA, 8.4×104 M atm−1) at 298±2 K. The effective derived gas-aerosol partitioning coefficient (KmP, 3.8×10−3 m3 µg−1) was 4 orders of magnitude higher on average than the theoretical value (KtP, 2.8×10−7 m3 µg−1) at 270±4 K. Beyond following Henry's law or Pankow's absorptive partitioning theory, the partitioning of H2O2 in the gas-liquid and gas-aerosol phases in the ambient atmosphere was also influenced by certain physical and chemical reactions. The average concentration of liquid-phase H2O2 in rainwater during summer was 44.12±26.49 µM. In 69 % of the collected rain samples, the measured level of H2O2 was greater than the predicted value in pure water calculated by Henry's law. In these samples, 41 % of the measured H2O2 was from gas-phase partitioning, while most of the rest may be from residual H2O2 in raindrops. In winter, the level of aerosol-phase H2O2 was 0.093±0.085 ng µg−1, which was much higher than the predicted value based on Pankow's absorptive partitioning theory. The contribution of partitioning of the gas-phase H2O2 to the aerosol-phase H2O2 formation was negligible. The decomposition/hydrolysis rate of aerosol-phase organic peroxides could account for 11 %–74 % of the consumption rate of aerosol-phase H2O2, and the value depended on the composition of organic peroxides in the aerosol particles. Furthermore, the heterogeneous uptake of HO2 and H2O2 on aerosols contributed to 22 % and 2 % of the aerosol-phase H2O2 consumption, respectively.