Xin Fang, Min Hu*, Dongjie Shang, Rongzhi Tang, Linlin Shi, Tinja Olenius, Yujue Wang, Hui Wang, Zijing Zhang, Shiyi Chen, Xuena Yu, Wenfei Zhu, Shengrong Lou, Yan Ma, Xin Li, Limin Zeng, Zhijun Wu, Jun Zheng, and Song Guo
https://pubs.acs.org/doi/10.1021/acs.estlett.0c00270
Abstract
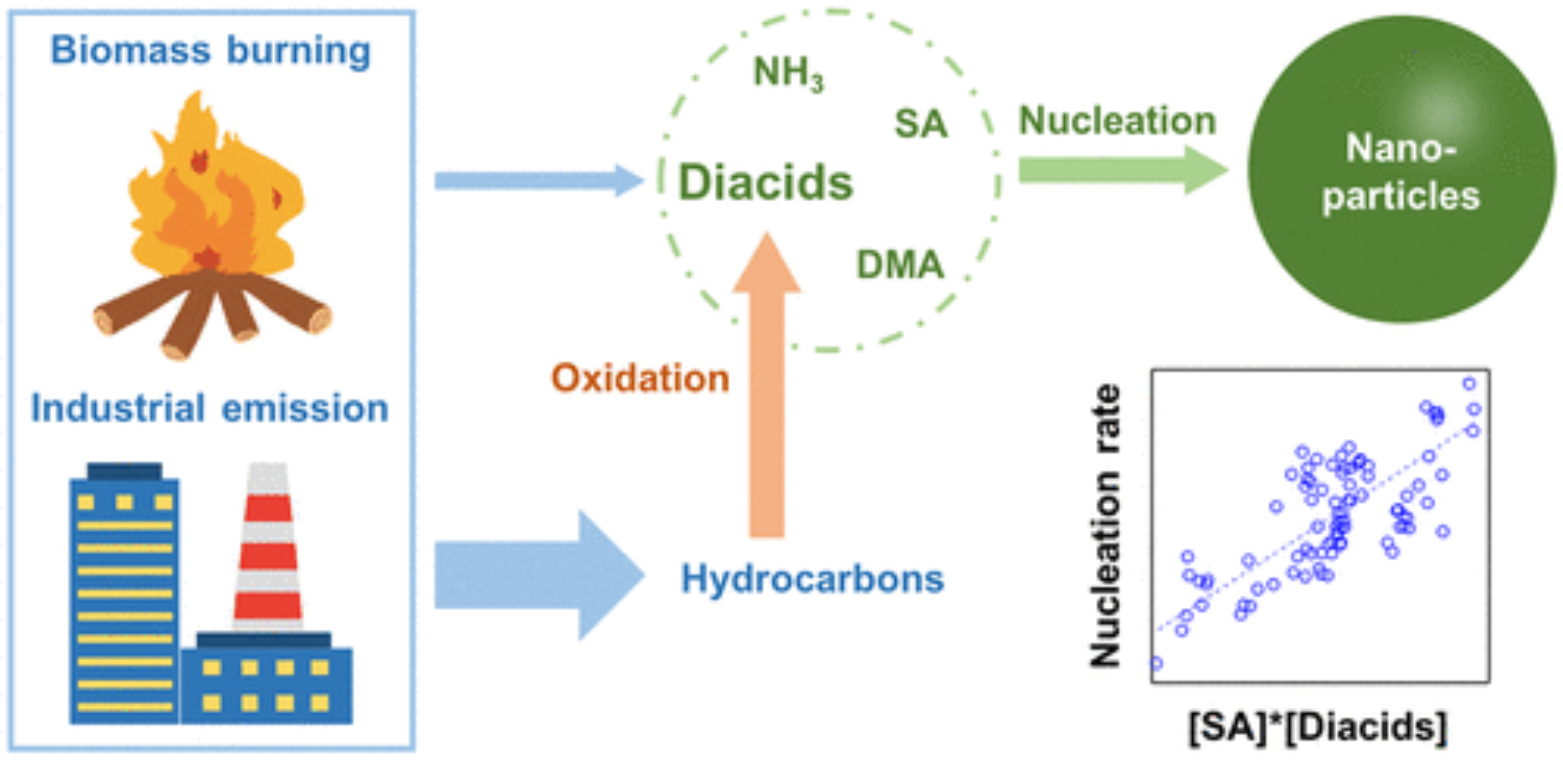
Gaseous dicarboxylic acids (diacids) are suggested to participate in atmospheric new particle formation via bonding with sulfuric acid (SA), ammonia (NH3), amines, and other molecules. However, there is a lack of observational evidence for the involvement of diacids in particle nucleation. Comprehensive measurements were conducted at a rural site of the North China Plain in winter, and unexpectedly high nucleation rates (JOBS, 30.5–839.7 cm–3 s–1) were observed under low SA levels (0.7 × 106 to 4.4 × 106 cm–3). Neither SA-NH3 nor SA-dimethylamine (DMA) mechanisms could fully explain the JOBS. Gaseous diacid monomers and dimers and diacid-SA-DMA clusters were identified in this study. The JOBS values were enhanced by a factor of 5 to 10 as the signal intensities of diacids increased 4-fold. Products of diacid signals and SA concentrations showed a positive correlation with the JOBS (Pearson’s correlation coefficient = 0.72). Experimental evidence was found that succinic acid competes with the second SA molecule for addition to the SA·DMA cluster. The concentrations of diacids were estimated to be 1–3 orders of magnitude higher than those of SA. We propose that diacids could actively participate in particle nucleation and may dominate the initial steps under high [diacids]/[SA] ratios.