Anxu Sheng, Xiaoxu Li, Yuji Arai, Yuefei Ding, Kevin M. Rosso, and Juan Liu*
Publication Date: May 18, 2020
https:// DOI: 10.1021/acs.est.0c00996
Abstract
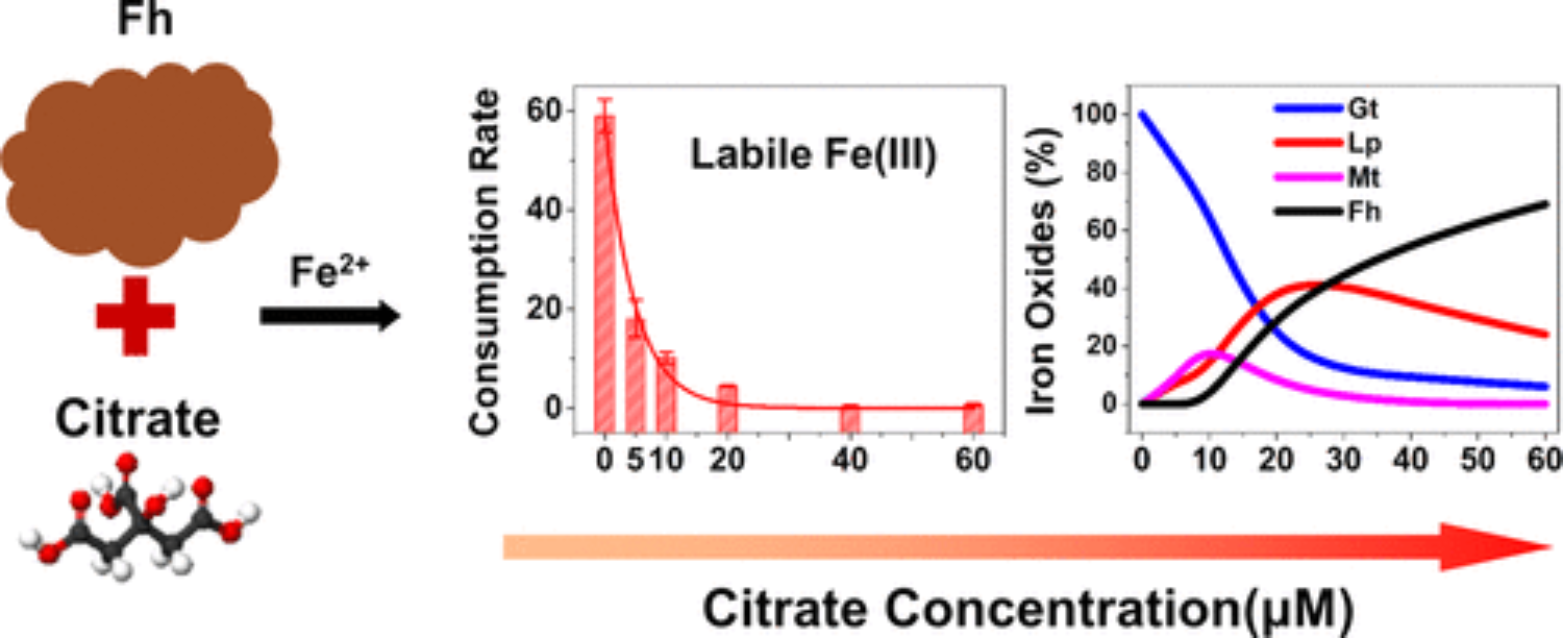
Ferrihydrite (Fh) is generally associated with dissolved organic matter (DOM) in natural environments due to a strong sorption affinity at circumneutral pH and its high specific surface area. In suboxic conditions, aqueous Fe(II) (Fe(II)aq) can catalyze transformation of Fh into more stable crystalline Fe(III) phases, but how DOM influences the transformation kinetics and pathway is still unclear. Using citrate as a surrogate, we have examined Fh transformation with 1 mM Fe(II)aq and 0–60 μM citrate at pH 7.2. We focus on quantifying the time-dependent concentrations of sorbed Fe(II), structural Fe(II), and a key intermediate species, labile Fe(III) (Fe(III)labile), resulting from interfacial electron transfer (IET), and how these species correlate with the evolution of lepidocrocite (Lp), magnetite (Mt), and goethite (Gt) products. Low concentrations of citrate significantly impact the proportions of Lp/Gt, and the collective results reveal that its effect is primarily through its ability to complex labile Fe(III) and thereby disrupt polymerization into product crystallites, as opposed to modifying the surface properties of Fh or inhibiting IET. The emergence of a Mt coprecipitate is observed in the transformation experiments with 5–10 μM citrate, when the Fe(II)/Fe(III)labile ratio on/near the Fh surface is close to 0.5, the stoichiometric Fe(II)/Fe(III) ratio in Mt. At the molecular level, the findings suggest that citrate, and by extension DOM, can modify the relative rates of olation and oxolation reactions that assemble labile Fe(III) into various product minerals.